As the world races to reduce the spread of the SARS-CoV-2 coronavirus, which causes COVID-19 disease, scientists warn that these strategies alone will not be enough. A study in The Lancet estimated that only four independently introduced cases of infection in a population are enough to make the probability of a large epidemic greater than 50%. Thus, experts say we should expect successive waves once the measures are relaxed. “Without an effective vaccine, I don’t know how this will end before millions of infections,” epidemiologist Nathan Grubaugh of Yale University told Vox Media.
Unfortunately, that is a much more distant horizon than the public would like. The average length of time from when development begins on a new vaccine until it is marketed is 16 years. However, in just three months since the beginning of the spread of this new virus in the Chinese city of Wuhan, the first candidate vaccines have already begun to be tested on humans. While the long process of clinical trials lies ahead, this is an unprecedented achievement that we owe to new vaccine technologies.
Traditional vaccines are based on the use of inactivated pathogens, or their neutralised components or toxins (in the case of certain bacteria). Another classic tool is the attenuated pathogen, which through successive cultures in the laboratory has lost its dangerousness. These procedures are quite standardised, and the vaccines we generally receive fall into one of these categories. But they are also laborious processes, as each vaccine against a new pathogen requires a fresh start from scratch. However, new technologies are based on prefabricated platforms that can be easily adapted to a new virus, like a construction kit in which just a few parts need to be changed.
Generally, these construction kits are recombinant vectors, vehicles that contain genetic material into which some part of the virus is inserted. For example, researchers use a harmless virus disguised as the dangerous one they want to vaccinate against. This is the approach used for one of the first SARS-CoV-2 vaccines already being tested in humans, the one developed by the Chinese company CanSino Biologics and the Beijing Institute of Biotechnology, which is now in phase 1 of clinical trials to assess its safety.
A harmless virus disguised
The platform that CanSino used is a harmless version of adenovirus type 5, a classic cold virus, in whose DNA has been inserted the gene for the Spike (S) protein, which the coronavirus SARS-CoV-2 uses to invade human cells. The aim is for this recombinant virus, which has no ability to replicate, to infect the cells so that they can produce their own vaccine: a coronavirus protein that will stimulate the immune response. The vaccine, called Ad5-nCoV, has already demonstrated its safety and immunogenic capacity in preclinical animal trials, according to the company.
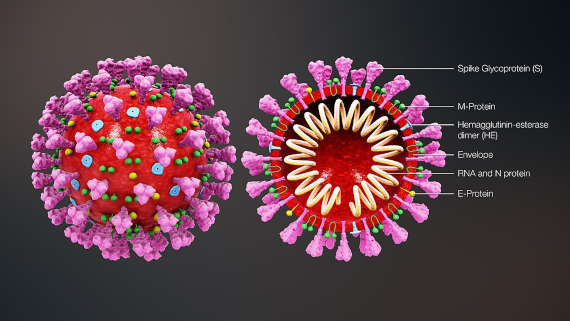
One of the biggest investments in the race to develop COVID-19 vaccines is also based on an adenovirus. Johnson & Johnson (J&J), through its subsidiary Janssen, is one of only two corporations among the top ten global pharmaceutical companies —along with Sanofi, which is using an baculovirus platform from insects— that have so far announced their vaccine projects, with a planned investment of $1 billion in which the US government is participating. J&J’s formulation is a recombinant, non-replicative adenovirus 26 with the S-protein gene of SARS-CoV-2. According to statements made to Science magazine by J&J Chief Scientific Officer Paul Stoffels, the vaccine is already being tested on animals. The goal is to begin a phase 1 trial in September, and move to a large-scale phase 2 trial with thousands of individuals next winter. The company aims to produce 300 million doses per year in a single facility, to which others will be added later.
DNA molecules of bacterial origin
Not all vectors that can transport the genetic material of the virus into cells are also viral in origin; some vaccines are based on the use of plasmids, DNA molecules of bacterial origin. Traditionally, plasmids have been used in research laboratories to introduce naked DNA into cells in culture using a technique called electroporation, which uses an electrical pulse to open small pores in the cell membrane. This procedure has now been adapted to vaccinations as well.
This is the approach used by the American company Inovio Pharmaceuticals, in collaboration with the Chinese company Beijing Advaccine. The adaptive power of these platforms to new vaccines is illustrated by the statements of Dr. J. Joseph Kim, Inovio’s president and CEO: the SARS-CoV-2 candidate vaccine INO-4800 was already designed on paper only three hours after the virus genome was published. After pre-clinical trials that the company has called “promising”, Inovio started a phase 1 trial on April 6 in which 40 people will be inoculated, and plans to have one million doses ready by the end of the year for trials and emergency use. The company already has a phase 2 vaccine against another coronavirus, the one responsible for Middle East Respiratory Syndrome (MERS).
The new era in vaccinology
An alternative to using DNA is to employ messenger RNA (mRNA), the intermediary molecule that cells use to convert genetic information stored in DNA into proteins. The idea is similar: providing the cell with instructions to make its own vaccine. This technology has been called “a new era in vaccinology” and “a universal strategy” against any emerging virus. This is the approach taken by Moderna Therapeutics and the US National Institute of Allergy and Infectious Diseases (NIAID), whose mRNA-1273 vaccine candidate is based on the mRNA of the S-protein. Moderna has already tested its platform on animals, which has allowed it to bypass this pre-clinical step and proceed directly to phase 1. On March 16, inoculations began on the first volunteers from a group of 45, making this vaccine the first in the West to enter clinical trials.
In total and according to information from the WHO dated April 4, there are now 62 candidate vaccines under development against SARS-CoV-2, employing a variety of strategies, including classical ones and those using proteins from the virus. But we should not lose sight of the fact that the vast majority of new platforms do not yet have approved vaccines. A long journey still lies ahead; as vaccine expert Florian Krammer and his collaborator Fatima Amanat of Icahn College of Medicine in Mount Sinai, New York, write in Immunity magazine, “vaccines may come too late to make an impact on the first wave of this pandemic. However, they might be very useful if additional waves occur later in time or in a post-pandemic scenario in which SARS-CoV-2 continues to circulate as a seasonal virus.” In any case, they add, it will improve our preparedness for the future, because “the viruses will keep coming.”
Comments on this publication