The SARS-CoV-2 coronavirus pandemic of COVID-19 has led to the unprecedented mobilisation of an industry whose role is key in this crisis: scientific research. The journal Nature estimated that by March 12, some 900 studies had already been conducted on a virus that was not even known three months earlier. Here we review the milestones science is reaching and the challenges still to be met in the battle against this global threat.
Preparing for epidemics
South Korea’s success in containing the COVID-19 epidemic without requiring the mass confinement of its population has been praised around the world. Studies have highlighted as key factors the implementation of mass testing, the isolation of those infected and the quarantine of their contacts, as well as controversial case tracking via mobile phones or credit cards. But this effective response is not the result of improvisation; following the outbreak of the Middle East Respiratory Syndrome (MERS) coronavirus, which in 2015 infected 186 people in Korea, 36 of whom died, the Korean government launched a special epidemic preparedness plan, which is now bearing fruit.

In contrast, other countries have been criticised for their lack of preparedness. In September 2019, the report “A World at Risk” from the Global Preparedness Monitoring Board, a body established by the World Health Organization (WHO) and the World Bank, warned of the risk of a pandemic caused by a deadly respiratory pathogen and of the low level of global preparedness, an alert that has proved to be prophetic. The SARS-CoV-2 pandemic is helping scientists improve epidemiological models, but it will be the responsibility of governments to listen to science in order to address this and future epidemics.
Diagnostic test
One of the achievements of science in the battle against SARS-CoV-2 is the development of the first diagnostic test in less than three weeks from the date of the WHO’s announcement of the outbreak in China, back when the virus did not even have a definitive name. In just a couple of months, there are already hundreds of diagnostic tests on the market or in development. The importance of these tools in the fight against the pandemic is underlined by a slogan oft repeated by Tedros Adhanom Ghebreyesus, the Director-General of the WHO: “Test, test, test.”

The most widespread tests are based on the detection of the genes of the virus by Polymerase Chain Reaction (PCR). Other tests are also coming on stream with the aim of lowering the cost and speeding up the diagnosis, rapid tests that do not require the samples to be sent to large automated centres, but can be carried out at medical care sites or even at home. Serological tests, which detect antibodies against the virus in the blood, are particularly important. They make it possible not only to diagnose the sick, but also to detect who has recovered from the infection even without symptoms, which experts say will serve to identify people already immune to the virus and to appreciate the true scale of the pandemic. Serological tests have already been used in Singapore and China, and are expected to be available soon for mass use in other countries.
New Antivirals
In the field of new virus-specific treatments, one approach is the use of monoclonal antibodies, molecules similar to those our immune system makes to fight infection, designed in the laboratory to target certain pieces of the virus. The structure of the Spike (S) protein that the virus uses to infect cells has been mapped out, a clear target for the design of these antibodies.
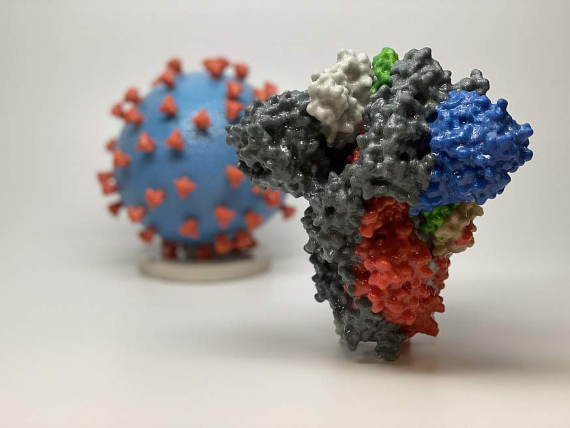
A second approach is to create drugs that interfere with the vital proteins of the virus. One study has revealed the structure of the main protease that SARS-CoV-2 uses to process the proteins that build new viral particles, along with that of an inhibitor that can block this function and prevent the virus from reproducing. However, any new treatment will have to undergo a long process of clinical trials, which means years of waiting.
Antisera and drug repositioning
Until the new drugs arrive, there are at least two other possible strategies. The first is to use plasma from people who have been infected and have recovered, and whose antibodies can help the ill fight against the virus. The second is to induce this immunity in animals and extract their serum, a technique invented in the late 19th century. This latter technique is a more rudimentary, though more immediate, version of the use of antibodies explained above, although its effectiveness is not guaranteed. This strategy has been used as the first line of control against Ebola, but reliable serological tests will be required before it can be applied to the new coronavirus.

A second approach is to make use of drugs already employed against other conditions and which may show effectiveness against the new virus. Hundreds of clinical trials following this path are now underway. Some of these compounds have failed in the initial tests, but there are hopes for others such as remdesivir, an experimental antiviral (not yet approved) created against Ebola and whose first results against SARS-CoV-2 are promising; or chloroquine, a classic medicine against the protozoan of malaria that can inhibit the infection. Both compounds are part of a large clinical trial project promoted by the WHO, focusing on four promising therapies. For its part, China has already approved for the treatment of COVID-19 the antiviral drug favilavir (or favipiravir), originally created by the Japanese group Fujifilm for use against other RNA viruses such as influenza. The advantage of drugs already approved for other illnesses is that repositioning them for use against COVID-19 is faster than the approval of a new drug.
The long-awaited vaccine
Even if progress in treatment succeeds in effectively combating the disease, epidemiologists stress that the end of SARS-CoV-2 lies in obtaining a vaccine. The effort in this area is also unprecedented; at the time of writing there are at least 50 candidate vaccines in development against the new coronavirus, but the list is growing almost daily.
Those leading the race are already beginning clinical trials. This is the case for the messenger RNA (mRNA) vaccine created by Moderna Therapeutics and the US National Institutes of Health. This formulation involves giving cells the instructions to make proteins from the virus that stimulate the immune response. The first healthy volunteers have already been inoculated in Seattle, from an initial group of 45. Another vaccine at this stage of clinical trials has been developed by the Chinese firm CanSino Biologics, using a viral vector formula that employs a harmless virus disguised with the proteins of the coronavirus. Inovio Pharmaceuticals will begin testing its DNA vaccine in humans in April, and others will soon follow suit. However, in this first phase only the safety of the vaccines is being evaluated. Experts and the WHO agree that, at best, completing all the phases necessary for the approval of a vaccine will still take between one and two years.
Comments on this publication