“Depending on your point of view, the clicking of the Geiger counter is either the heart-beat of the atomic age or the symbol of menacing radiation.” Thus began the tribute that the German University of Kiel paid ten years ago to one of its most illustrious professors, Hans Geiger, whose experiments helped other great physicists to bolster their revolutionary theories. He was one of the pioneers of the scientific study of something totally new —radioactivity— and with this work he completely changed our view of the atom. Although he was never recognised with the Nobel Prize, his marvellous invention earned him even greater fame than that of many prize-winners, and which still remains strong today.

The radioactivity counter of Hans Geiger (30 September 1882 – 24 September 1945), and its characteristic sound, starred in some of the most disturbing scenes of the recent television series Chernobyl, which recounts the disaster of the ill-fated Soviet nuclear power plant. The device was also the central thread of Geiger’s entire brilliant scientific career as an experimental physicist. The decision that set him on that path was to leave his native Germany —having recently completed his doctorate in 1906— to go on a fellowship to the University of Manchester, where he soon began collaborating with Ernest Rutherford, who had already identified the three main types of radioactivity: alpha rays, beta rays and gamma rays.
Rutherford was soon awarded the Nobel Prize in Chemistry (1908) for his research into the disintegration of radioactive elements, but he did not stop there. Further study of the alpha rays emitted in these radioactive decays led to one of the most amazing moments in the history of science: “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you,” Rutherford recalled years later at a conference. Actually, the first person to see it was Geiger, and it wasn’t exactly a “Eureka moment” that happened on a particular day. Rutherford had put him to work counting alpha particles, and it was in 1908 that Hans Geiger developed the first version of his counter, which turned out to be very unreliable as there were many anomalies. Rutherford’s study led Geiger and student Ernest Marsden to undertake a multitude of experiments over the next five years.
An amazing experiment
This is what is now taught in high school as the “Rutherford gold foil experiment”, but which encyclopaedias more rightly call the Geiger-Marsden experiment. While shooting an alpha particle beam at a thin gold foil, Geiger was stunned to see some of the particles bounce backwards. Rutherford deduced that the unexpected result of the experiment was only possible if atoms concentrated their mass in a tiny (positively charged) nucleus, and basically this is the idea of the atom we still have today.

Geiger’s experiment would bring Rutherford further renown and showed that radioactivity could be used to explore the inside of atoms and discover their internal structure, opening a new path for science. After these successes, Hans Geiger returned to Germany, where he ran his own laboratory and continued to improve his methods for counting radiation. Together with James Chadwick, who won the 1935 Nobel Prize for the discovery of the neutron, he improved his counter to also measure beta rays. After the interruption of the First World War, in which he served as an artillery officer, Geiger returned to his experiments and in 1924 continued working on his device. As a result, he was able to verify the Compton effect (a form of light-matter interaction, different from Einstein’s photoelectric effect), which was a fundamental help for Arthur Compton, who received the Nobel Prize in Physics in 1927.
From radioactivity to cosmic rays
By then Geiger had settled at the University of Kiel, in northern Germany, where he finished developing his invention with the assistance of a PhD student, Walther Müller. While his ingenious 1908 device was very impractical —it only detected alpha particles and the operators had to take turns observing with the naked eye very faint flashes in a completely dark laboratory— the definitive version of the Geiger counter was an efficient, reliable, durable and portable device, which also made it possible to detect many different types of ionizing radiation (not just alpha particles).
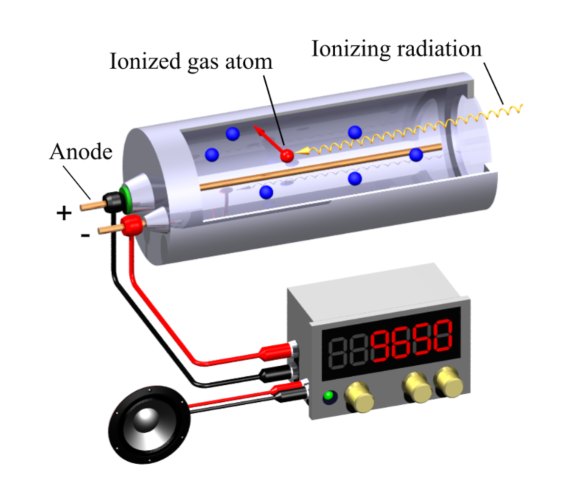
That model is the basis for the counters used today both to measure the risk of radioactive contamination and to determine the radiation therapy dose a patient will receive. They are also still used in scientific research, following in the footsteps of Geiger himself, who was able to detect a cosmic ray shower with his counter. Geiger dedicated the final stretch of his scientific career to the study of these particles, which travel through space at close to the speed of light. This new door to observe the cosmos was opened by Geiger’s enthusiasm for experimentation and his drive to improve his invention.
Comments on this publication