If ice cubes sank rather than floated, you might think this wouldn’t cause us much trouble; we’d just have to stir our drinks from time to time to keep the top part cool. This wouldn’t be so bad, except for the fact that there would be no drink to stir or anyone to do so, because there would be no life on Earth, or at least humans would not be here.
The fact that ice floats is one of the oddities of water, a substance so familiar that it seems ordinary, but there is nothing ordinary about it. So different are its properties from what one would expect from its chemical composition, that understanding how its microscopic structure causes such unique behaviour is something that “has been discussed intensively for more than 100 years and has not yet been resolved,” as physicists Lars Pettersson and Anders Nilsson, from the University of Stockholm (Sweden), and Richard Henchman, from the University of Manchester (United Kingdom) wrote in a recent review.
A crucial biochemical role
Every student knows that substances contract when cooled and expand when heated, which allowed the invention of the thermometer. Hot water also reduces its volume as it cools. But below 4° C something extraordinary happens: it begins to expand again, as everyone who has ever frozen a bottle that is too full knows only too well. The result is that ice is less dense than liquid water and therefore floats, a phenomenon that already interested Galileo Galilei back in 1612 when he reasoned that “ice should be rather water rarified than condensed“.
But what would happen if this were not so? In oceans, lakes and rivers, the floating layer of ice that forms in winter prevents heat from escaping, keeping the water beneath in a liquid state. If the ice sunk to the bottom, more ice would continue to form until everything became a large solid mass; the heat from the surface would only melt a thin upper layer, which would have made it impossible for complex life as we know it today to evolve. In addition to the crucial biochemical role of water, the biology, geology and dynamics of the oceans were fundamental in making the Earth a habitable planet.

This is by no means the only unusual property of water. From its chemical formula, as hydrogen dioxide (H2O), it should follow the pattern set by hydrogen sulphide (H2S), hydrogen selenide (H2Se) or hydrogen telluride (H2Te), similar compounds with the elements that follow oxygen in its group of the periodic table. If this were so, then water should boil when warmer than -80° C and freeze at -100° C. Fortunately for terrestrial life, we know that this is not the case; the fact that it freezes at 0° C and boils at 100° C —at our normal atmospheric pressure— not only gives it a wide range of temperatures in a liquid state, but also makes it the only substance that under the living conditions of Earth is found in solid, liquid and gaseous form.
A weird physical-chemical phenomenon
It can be inferred from the above that the aberrant properties of water are, as chemist Martin Chaplin, professor emeritus at the London South Bank University, confirmed to OpenMind, “totally responsible” for the existence of life on Earth. In fact, adds Nilsson, curiously “it looks like water becomes anomalous at temperatures where life seems to exist most of the time.” But what kind of weird physical-chemical phenomenon should we thank for our existence? According to Chaplin, “there are several explanations, but none have proven to be conclusive or all embracing.” At its origin are the peculiar characteristics of oxygen, one of the most electronegative elements of the periodic table. When combined with hydrogen it attracts the electrons to itself with such force that the water molecule, although electrically neutral as a whole, forms two poles, positive and negative.

This dipolar nature of water is key, as it allows it to form hydrogen bonds. Compared to other seemingly similar compounds, “the hydrogen bonding in water is much stronger and more extensive,” says Chaplin. These bonds give water a sticky behaviour that is responsible for its enormous surface tension —the highest in any liquid except mercury— which can act as both a lubricant and an adhesive between surfaces.
Intense scientific debates
It is the microscopic structures derived from these links, which in turn determine the anomalous properties, that still keep scientists engaged in intense debate. Ice has a stable tetrahedral structure, with one water molecule in the centre joined by hydrogen bonds to another four at the vertices. This spacious regular arrangement is the cause of the low density of frozen water. It used to be thought that as it went into the liquid state, this structure simply became more dynamic, breaking down and forming hydrogen bonds at a rate of one billion times per second, resulting in a more compact mass.
But in 2004, Nilsson, Pettersson and their collaborators discovered that the molecules in liquid water tend to leave the tetrahedral structure and form only two hydrogen bonds. “We proposed that the dominant structure is severely distorted,” says Nilsson. “Since then we have also put forward that there are two fluctuating structural domains, high and low density liquid.” The model states that liquid water does not form a homogeneous structure, but is a mixture of two different ones: a light tetrahedral and a dense and disordered one that predominates at ambient temperatures. This arises when ice melts, increasing its density, but above 4° C the increase of the dense form induces a repulsion between the molecules that results in thermal expansion.
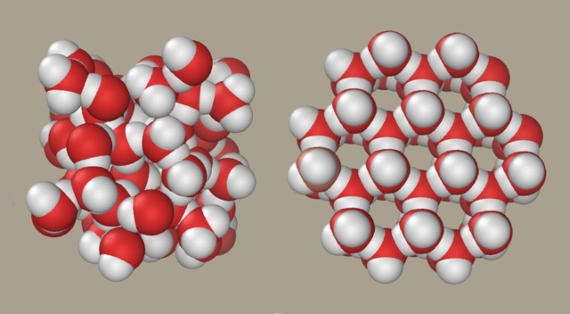
Thus, according to the model of Nilsson and Pettersson, these two different and rapidly changing structures coexist in the same liquid under terrestrial environmental conditions. At extreme temperatures or pressures, water adopts only one of the two configurations and behaves like any other liquid, without those oddities that make it the strangest liquid in the universe and to which we owe our existence.
Javier Yanes
Comments on this publication