There is no technology at present that generates the expectations that are being created by solar cells based on a class of materials called perovskites a true revolution in the field of photovoltaic solar energy. Let’s try to understand the reasons
How do solar cells harness the Sun’s energy?
Just before entering the Earth’s atmosphere, solar radiation is mostly distributed in wavelengths between 200 nm (ultraviolet) and 2500 nm (infrared), as shown below:
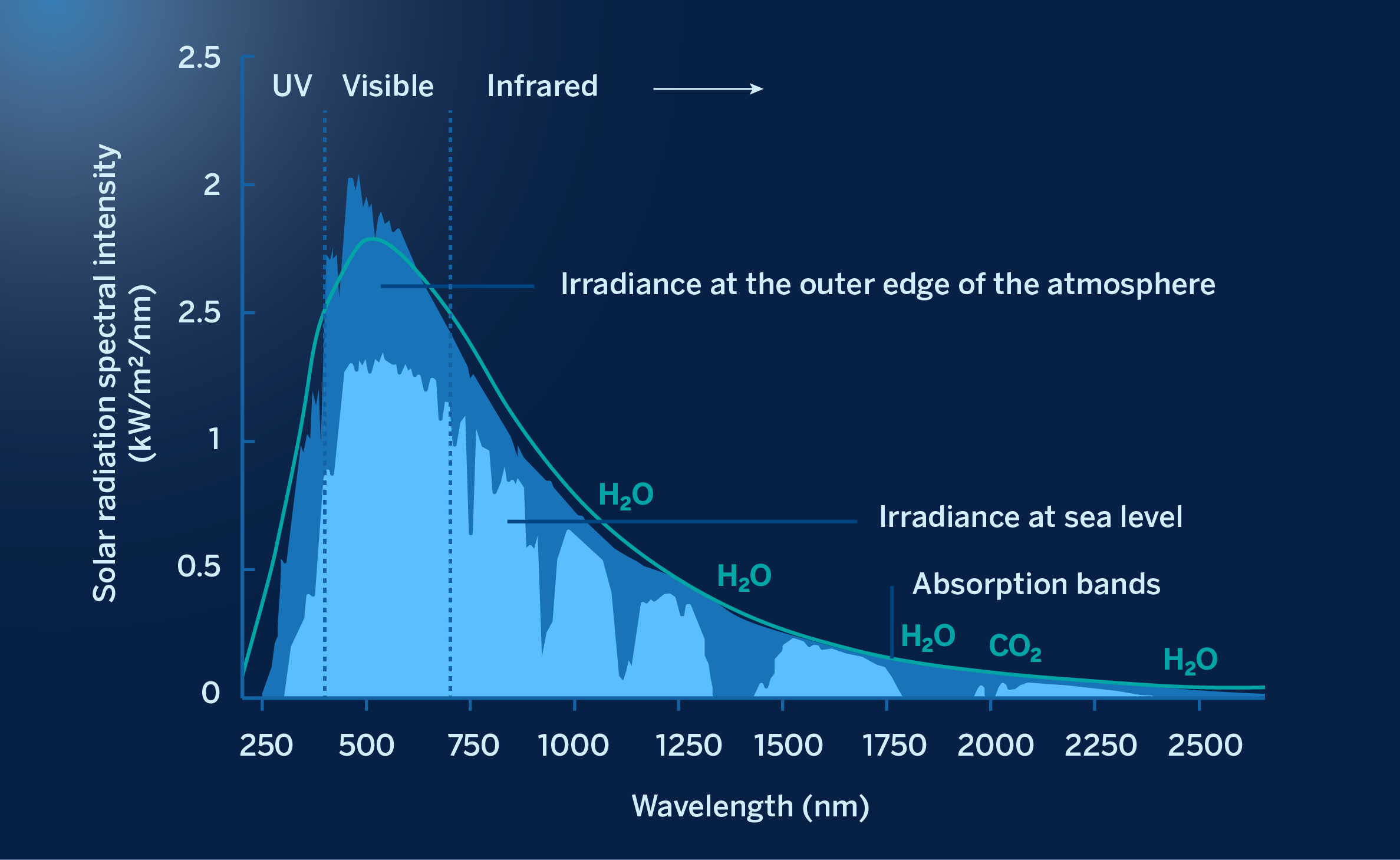
The spectrum of the Sun at the outer edge of the atmosphere (in yellow) and at the earth’s surface (in red)
As can be seen in the figure above, a large part of this radiation does not reach the Earth’s surface for several reasons. A part is dispersed by dust, aerosols and various molecules suspended in the atmosphere, another fraction is returned to outer space by reflecting off clouds, which play an essential role in regulating the temperature of the atmosphere and the Earth’s surface. There is also a significant part that is absorbed by atoms and molecules in suspension: nitrogen and oxygen absorb the ultraviolet, blocking the arrival at the surface of these radiations, with wavelengths below 200 nm. Furthermore, oxygen molecules can break down after absorbing oxygen, giving rise to ozone molecules, which in turn absorb even shorter wavelength ultraviolet radiation.
All these processes produce deep “trenches” in the spectral distribution of radiation, as shown in the figure above. For example, much of the infrared radiation of a wavelength greater than 2000 nm is absorbed by water vapor (H20) and C02. Similarly, most of the ultraviolet light, of wavelengths shorter than 300 nm, is absorbed by the ozone, although not enough to completely prevent sunburn.
When sunlight strikes a semiconductor, it only absorbs light of an energy greater than a magnitude known as the “bandgap”, a characteristic parameter of each semiconductor obtained from quantum physics applied to solids, and which is expressed in electron-volts (eV). Not all energies above the bandgap are efficiently absorbed by a semiconductor; on the contrary, the absorption of these energies produces undesirable phenomena in the solar cell made with a given semiconductor. The following figure illustrates this for a silicon solar cell, the current dominant technology in the photovoltaic industry today.
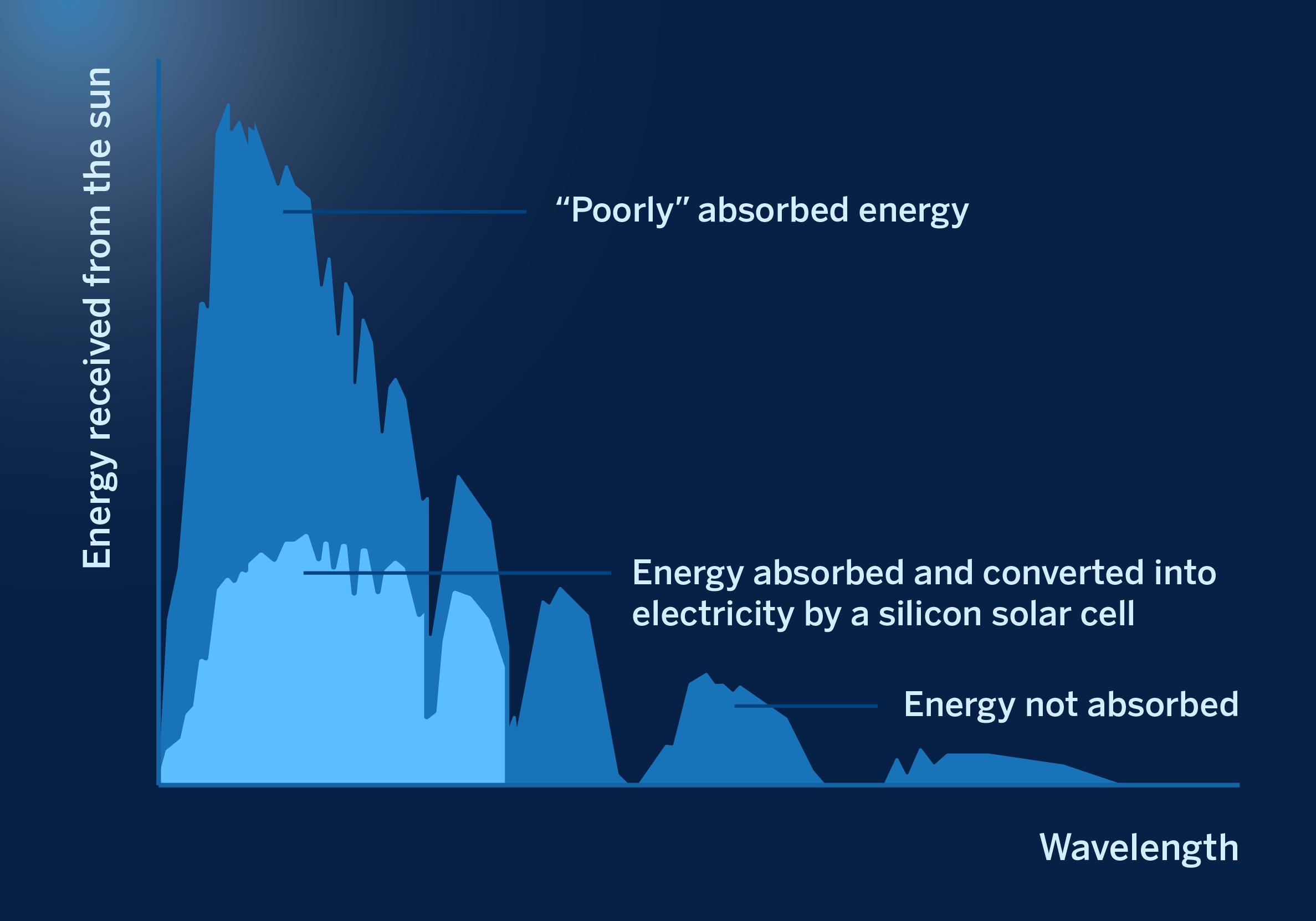
Energies of the solar spectrum that are adequately absorbed by a silicon solar cell (yellow) and those that are not absorbed or are absorbed inefficiently (gray)
From a theoretical point of view, the maximum conversion efficiency of a solar photovoltaic cell from solar energy into electrical energy of a photovoltaic solar cell is obtained for a device made of semiconductor in which the value of its forbidden band is 1.4 eV, a calculation that has been known for decades and which we owe to W. Shockley and H.-J. Queisser which sets an absolute limit of 32.5% on the efficiency of a photovoltaic cell composed of a single absorber layer. To obtain higher solar-to-electrical conversion efficiencies, absorbing a more significant fraction of the sunlight more efficiently is essential. This is achieved by stacking several cells with different forbidden band values on top of each other, in structures called tandem (a junction of two cells) or multi-junction (a junction of three or more cells). With these structures, the theoretical efficiency limit can be raised to 44.3% (tandem) and 50.1% for triple junction structures.
We shall now have a look at what perovskites are and why they are so interesting to use as devices capable of efficiently converting solar energy into electrical energy.
What are perovskites?
Perovskite is a naturally occurring mineral called calcium titanate the chemical formula of which is CaTiO3. This mineral was first discovered by the German mineralogist Gustav Rose in 1839 and was named after the Russian mineralogist Lev Perovski. Generally, materials that have the same crystal structure as CaTiO3 are called perovskite materials. In general, they have a generic composition of the form AMX3, where A and M are metal cations and X is an anion. The way in which the different atoms of the compound are grouped in space is shown in the following figure:
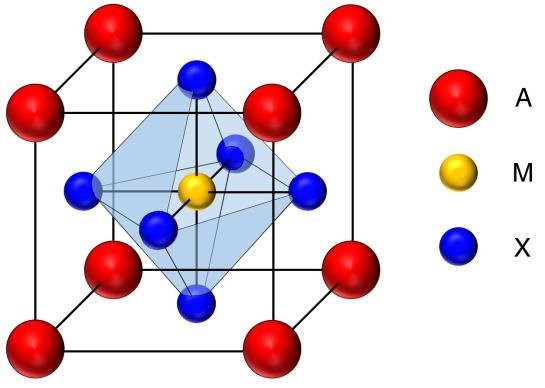
Generic crystalline structure of perovskite materials
In recent years, a group of perovskite materials named organic-inorganic hybrid perovskites with a more complex chemical composition, consisting of an organic ammonium cation A (CH3NH3, NH=CHNH3, etc.), a metal cation B (Pb, Sn, Cd, Fe, Co, Cu, etc.) and halide ions X (Cl, Br or I), has attracted substantial interest. Since the 1970s, much research has been conducted on this family of materials to better understand the diversity of optical, magnetic and electronic properties they possess. Thanks to these properties, organic-inorganic perovskites are ideal for making photovoltaic devices, although this has only become apparent well into the present century. Indeed, perovskite-based solar cells have experienced unprecedented growth among new-generation photovoltaic technologies, with certified efficiencies in excess of 25% today in single cells. Moreover, since the perovskite composition can be adjusted to obtain variable energy bandgap values over a wide range, silicon tandem cells with efficiencies above 30%, such as the one seen at the start of this article, can be made. In addition, the ease of obtaining perovskite solar cells allows for them to be manufactured in industrial quantities with low investments, which is important to offset the additional costs of tandem solar cells in a short period of time by increasing the power output obtained compared to single cells.
The brief history of perovskite-based solar cells
The first perovskite-based photovoltaic device was published in 2009 in which methylammonium lead iodide (CH3NH3PbI3) and methylammonium lead bromide (CH3NH3PbBr3) were used. It was advertised as having a low conversion efficiency (3.8%) and very low stability, which is why it was of little interest.
In 2012, the characteristics of the sorbent material (CH3NH3PbI3) were improved, as a result of which conversion efficiency was increased to 10%. These results, obtained in such a short period of time, triggered a worldwide “perovskite fever”. In November 2014, a device manufactured by researchers at the Korea Research Institute of Chemical Technology (KRICT), achieved a record unstabilized efficiency of 20.1%. In December 2015, researchers at the École Polytechnique Fédérale de Lausanne (EPFL) achieved a record 21%. In March 2016, researchers from KRICT and the Ulsan National Institute of Science & Technology (UNIST) achieved a new record of 22.1%. Today, this efficiency is already at 25.6% for a single cell competing on an equal footing with silicon solar cells (albeit with stability issues still unresolved). No other PV technology has achieved a succession of efficiency records at this speed. The figure below shows some important milestones in the progress of perovskite-based photovoltaic devices:
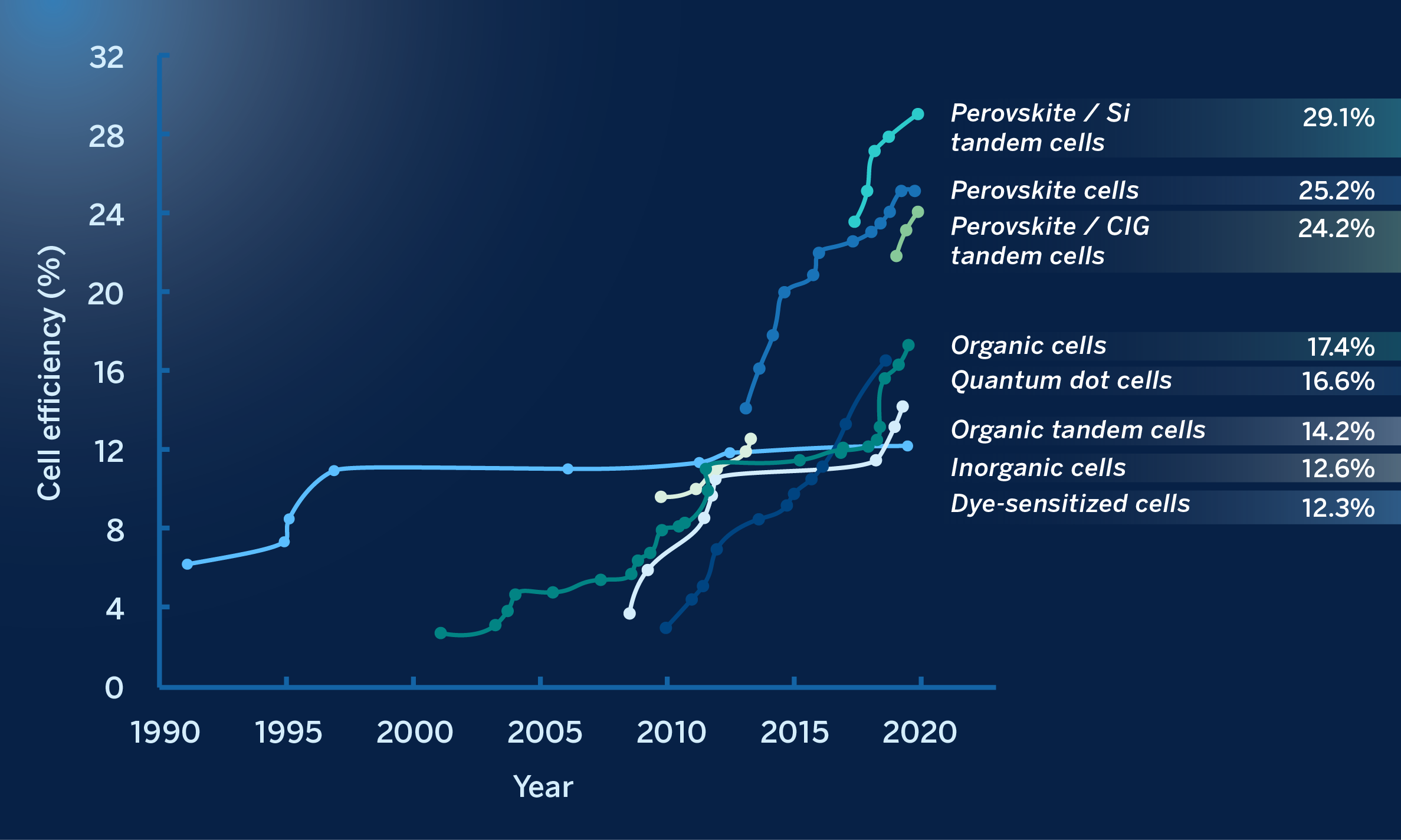
The race for the efficiency of various perovskite-based solar cells, compared to other emerging technologies. As indicated at the beginning of this article, the 30% barrier has recently been surpassed.
This blazing progress has stimulated a great deal of interest in this PV technology, as evidenced by the fact that the number of articles published annually on the subject is increasing year after year. The figure below shows this progression, measured as the number of annual publications from 2009 to last year:
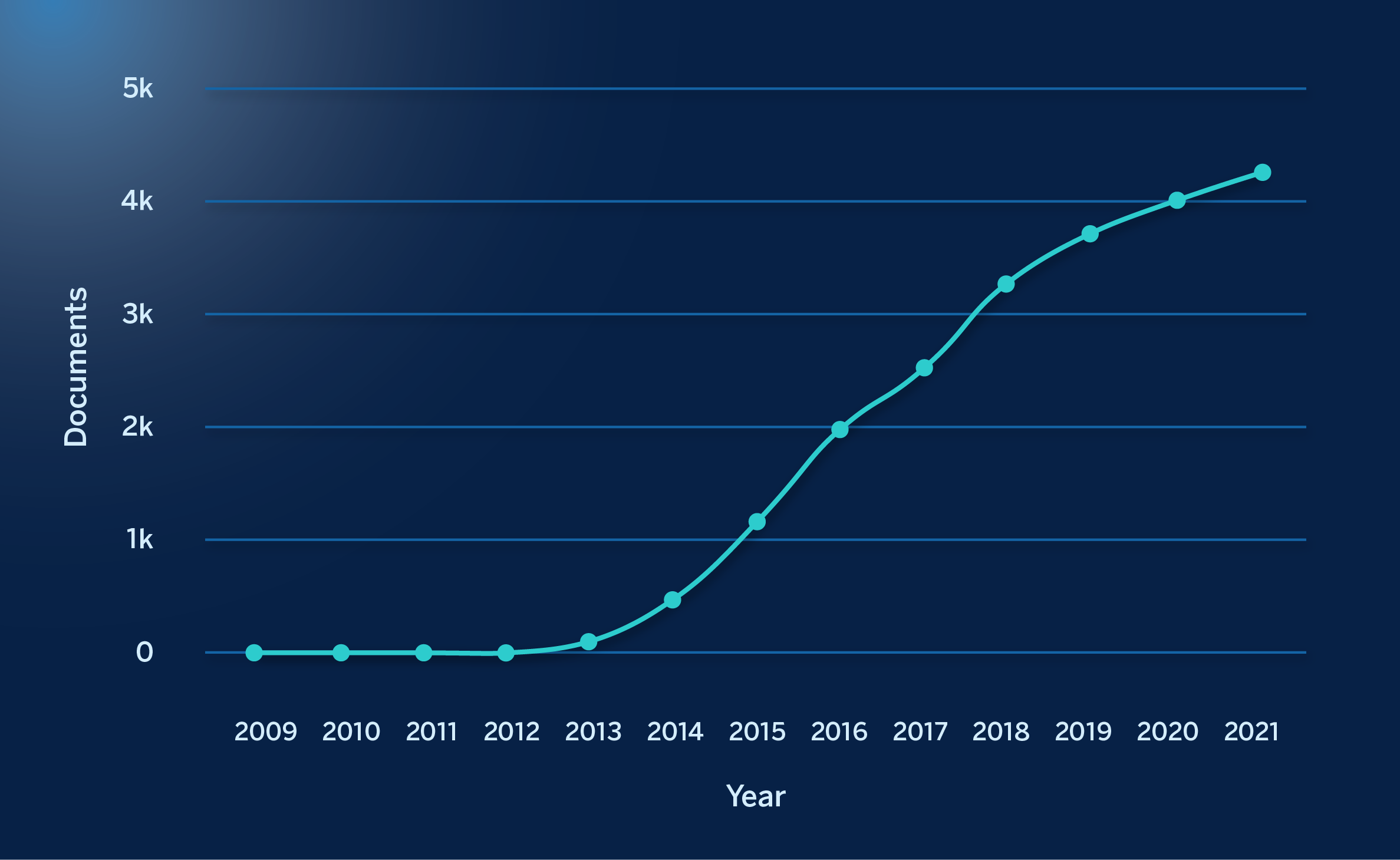
Search for “perovskite solar cells” in the Scopus portal at the beginning of October of this year, showing the number of scientific articles published on these devices from 2009 (21) to the end of last year, which exceeded 4,200 articles.
In a forthcoming article, I will describe what is arguably the best hope for obtaining very high efficiency values in cost-competitive photovoltaic devices today: silicon-perovskite tandem cells.
Comments on this publication