In late 2020, it was discovered that the wildfires that had devastated Australia a year earlier had caused significant damage to the ozone layer in the southern hemisphere. Now, a study by researchers at the Massachusetts Institute of Technology (MIT) has identified the mechanism by which this alarming destruction is taking place, a warning of what can happen on an overheated planet where fires are becoming more frequent, longer-lasting and more massive.
As well as devouring millions of hectares of forest, huge pyrocumulonimbus clouds—the technical name for plumes of smoke that reach heights of several kilometres—rose into the stratosphere, injecting millions of tonnes of aerosols. Months later, satellite data revealed that this smoke had somehow depleted part of the ozone layer. Specifically, between 3% and 5% of the ozone present in the mid-latitudes of the Southern Hemisphere, in the belt located over Australia, New Zealand and regions of Africa and South America. In addition, after reaching the polar regions on stratospheric air currents, the smoke caused the destruction of ozone at the edges of the ozone hole over Antarctica, expanding it by 2.5 million km2—10% of its total size before the event.
The findings of the data collected prompted MIT scientists to investigate the mechanism by which smoke from the wildfires caused this massive loss of ozone. The mechanism has now been identified, thanks to a study whose initial results were already predicted just one year ago.
In order to understand the destructive chemical process, the first step was to re-examine what was already known: how ozone depletion occurs at the polar vertices, and particularly over the South Pole.
The example of the South Pole
It all starts with chlorofluorocarbons (CFCs), which have been banned since the 1990s but were used for a long time and emitted into the atmosphere on a massive scale. CFCs are highly persistent compounds, but they are also very photoreactive under the influence of intense ultraviolet radiation reaching the upper layers of the atmosphere. Over time, these CFCs are degraded and break down, leading to the formation of other chlorinated compounds, most notably hydrochloric acid (HCl).
While hydrochloric acid in its gaseous state is not very reactive and therefore harmless to ozone, the problem arises when something causes this HCl to decompose, because in this state it has a strong tendency to react with the oxygen atoms of ozone (O3). In the polar regions, the dissociation of HCl occurs when it is absorbed by the nitric acid and water droplets contained in stratospheric clouds. When absorbed on the surface of these droplets, the conditions are right for the hydrochloric acid to dissolve and the chlorine atom to be released.

The presence of these clouds is restricted to polar latitudes, where the right temperature and low humidity conditions exist for their formation, conditions that are not present at lower latitudes. With this process in mind, the researchers turned their attention to wildfire-derived aerosols to see if, somehow, they also triggered an analogous reaction.
The ozone-destroying molecule
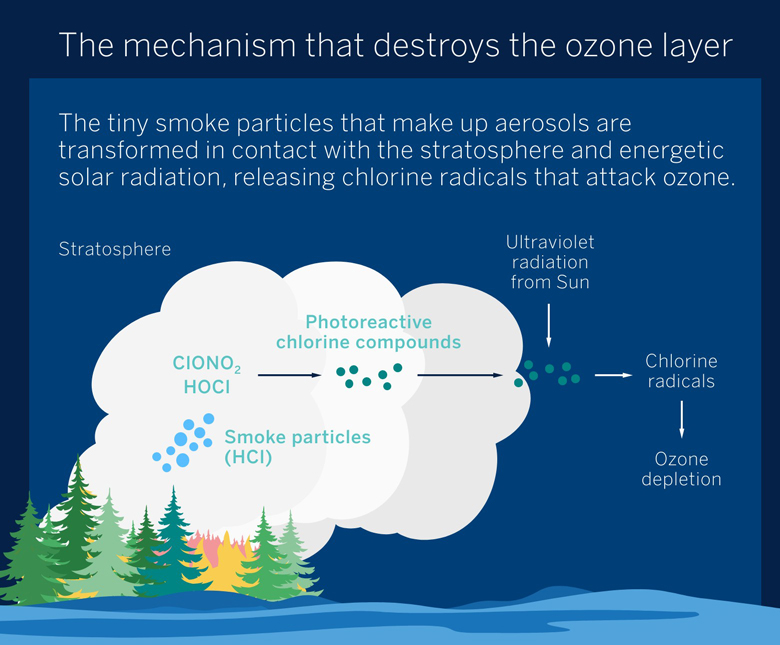
The key here lies in the organic nature of the tiny smoke particles that make up these aerosols. Because of their plant origin, these particles contain organic acids capable of dissolving hydrochloric acid and weakening the H-Cl bond sufficiently to allow the chlorine atom to be released.

As they rise, the aerosols absorb water molecules on their surface, and when they reach the stratosphere they absorb the HCl present. The combination of water and organic acids creates the conditions for the HCl to dissolve, weakening the molecular bond sufficiently for the chlorine atom to react with other chlorinated compounds in the stratosphere and with derivatives of CFCs, such as hypochlorous acid (HClO) and chlorine nitrate (ClNO3). This gives rise to photoreactive compounds that break down under the action of the energetic solar UV radiation present at these altitudes, releasing Cl radicals (free chlorine atoms with an enormous zeal for reacting with other atoms).

These chlorine radicals are ultimately what attack the ozone (O3), leading to the formation of molecular oxygen (O2) and chlorine monoxide (ClO), a compound dubbed the ozone-destroying molecule because of its extreme reactivity. The oxygen in ClO immediately reacts with the free oxygen atoms present in the stratosphere to form O2, releasing the Cl radical, which destroys yet another ozone molecule in a chain reaction.
Comments on this publication