Alejandro Ortiz became completely deaf due to meningitis when he was two and a half years old. The infection spread to his inner ears, where it destroyed specialised cells in charge of transforming the mechanical impulse of sound into the electrical impulse of the auditory nerve. By the age of four, Ortiz could hear again, but the sensation was unfamiliar. Sound didn’t arrive at his brain through the ear; rather, it entered via a small microphone attached to the side of his head, which relayed radio signals to minute electrodes implanted in his auditory nerve. “The cochlear implant had a great impact on my life,” says Ortiz, now a young Barcelona resident who can hear and speak with fluidity.
Just a few decades ago, the direct and controlled stimulation of the nervous system belonged in the realm of science-fiction. In fact, many of the brain’s inner workings are still a mystery to scientists and, therefore, they pose a challenge for physicians when something goes wrong. But with the advent of neurotechnology, based on the physical connection of machines to the nervous system, doctors have achieved wonders beyond the reach of pharmacology. The new techniques have already made it possible to moderate the symptoms of Parkinson’s disease and epilepsy, to speed up the rehabilitation of patients who have suffered a stroke, and are even beginning to fulfil the hope of restoring mobility to people with paraplegia and quadriplegia.
The cochlear implant, which benefits several hundred thousand hearing-impaired people (736,900 devices have been implanted worldwide as of December 2019), is now familiar to us because of its popularity. Approved for therapeutic use in the 1980s for adults and at the beginning of this millennium for children, it was one of the first medical achievements of neurotechnology and the second bionic device after cardiac pacemakers. But it’s not a perfect solution, as the implanted electrodes lack the fine-tuned resolution of cochlear cells required to distinguish every sound frequency in the audible spectrum. Noisy environments, rooms with reflective surfaces and multiple speakers can make hearing challenging for users of these devices.
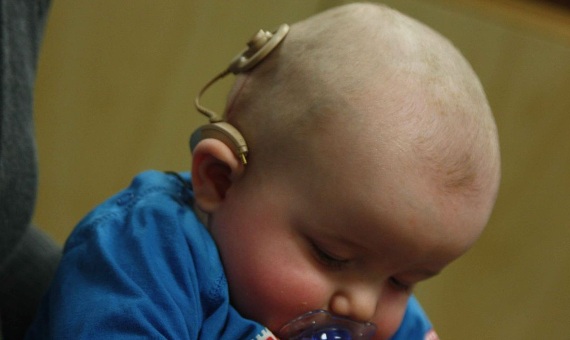
In addition, Ortiz—like all those who have received the implant—had to relearn how to hear. During his childhood and adolescence he went to various specialists and rehabilitation centres to train his brain to interpret the electrical impulses generated by his cochlear implant. He encourages anyone who could benefit from it to undergo the operation as soon as possible: “Language is developed faster in childhood,” he explains.
If cochlear implantation can restore hearing to those who have lost it, it is also possible to do something similar with sight in patients who have a functional optic nerve but have suffered degeneration of the photoreceptors in their retina, which occurs in genetic diseases such as retinitis pigmentosa or choroideremia. Artificial retinas began to be developed in the 1990s based on the same principle as the cochlear implant: picking up sensory stimuli from the environment—in this case visual stimuli—via a sensor and transmitting them to the nerve responsible for sending them to the brain.
The first device of this type, called Argus II, was approved in the 2010s in Europe and the US. It consisted of a digital camera mounted on a pair of glasses that sent the images to a processor, which converted them into electrical signals that were sent via wires to electrodes implanted in the retina. The results were not optimal and the product was discontinued in 2020, but there are many other projects underway for different types of artificial retinas. At the moment, these visual prostheses are not able to replace natural sight, but only provide pixelated images that allow people to distinguish light, shadows and some shapes, but the technology is advancing rapidly.
Cochlear implants were one of the first medical applications of neurotechnology, approved for therapeutic use in adults during the 1980s and for children at the turn of the century. Today, hundreds of thousands of people with a hearing disability benefit from them (324,000 estimated users in 2012). It’s not a perfect treatment, because the implanted electrodes lack the fine-tuned resolution of cochlear cells, required to distinguish every sound frequency in the audible spectrum. Noisy environments, rooms with reflective surfaces and crowded conversations make hearing difficult for users, too.
In addition, everyone who bears the implant must learn to listen from scratch. Ortiz attended hearing school and several specialists during his childhood and adolescence to train his brain in the interpretation of the electrical signals generated by his cochlear implant. He urges anyone who can benefit from the device to take the step as early as possible. “Language is developed faster in childhood,” he says.
Recreate the sense of touch
If sight and hearing can be restored, although there is still a long way to go, why not touch? People with spinal cord injuries lose not only mobility but also sensation in their limbs, and this is another case where bionic devices can help. It is even possible that amputees could also have prostheses with the ability to transmit the sensations of touch and pressure.
For patients with spinal cord injuries, an attempt is being made to open a pathway that bypasses broken neural connections, so that skin sensations can be transmitted by wires to the brain regions responsible for sensing them. In 2016, the team led by Robert Gaunt at the University of Pittsburgh succeeded in enabling a 28-year-old quadriplegic patient to regain sensations of touch in four fingers of his own paralysed hand, thanks to electrodes placed in the sensory region of the brain that were connected by wires to a prosthetic arm. In 2020, a patient’s own hand was able to send touch signals to the nervous system, thanks to a set of electrodes on the skin and a microprocessor implanted in the brain.
Bionic prostheses with sensitivity also hold promise for people who have lost limbs. In 1998, the first bionic arm controlled by electrical impulses was implanted in a patient, and since then the technology has advanced enough to provide these prostheses with touch sensations to improve the control. In 2014, a Danish patient who had lost his left hand received a prosthesis capable of sensing touch for the first time, thanks to pressure sensors that sent a signal to his nerves.
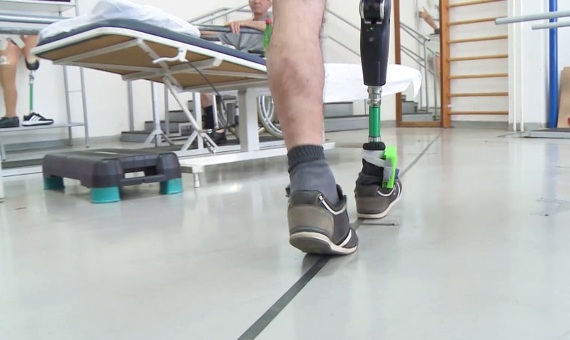
In 2019, an international team of scientists led by researchers at the EPFL centre in Switzerland succeeded in recreating the sense of touch in the prosthetic legs of three patients with above-knee amputations. All of them regained the ability to walk around obstacles intuitively and without looking at the ground.
“After all of these years, I could feel my leg and my foot again, as if it were my own leg,” Djurica Resanovic, who lost his right leg above the knee in a motorcycle accident, reported to the media after his procedure. “It was very interesting. You don’t need to concentrate to walk, you can just look forward and step. You don’t need to look at where your leg is to avoid falling.” This technology sends information directly to the nervous system, like a cochlear implant. The way all these neuroprostheses operate is the reverse of other brain-machine interfaces that “read” brain commands to operate an external device, such as a mind-controlled wheelchair.
Like other devices of this type, Resanovic’s bionic leg is equipped with pressure and movement sensors, which send wireless signals to electrodes permanently inserted in the tibial nerve of his stump. Resanovic could feel the prosthetic leg as an extension of his body: “I could tell when they touched the [big toe], the heel, or anywhere else on the foot. I could even tell how much the knee was flexed,” he reported. Clinical trials have demonstrated that this sort of bionic technology reduces fatigue and the appearance of phantom pain frequently linked to limb amputations. At the current rate of progress in this field, some experts are confident that within the next decade, amputees will be able to have prostheses that give them a completely natural experience and control, as if they were their own limbs.
Bidirectional communication
Since the 1980s, direct electrical stimulation of the nervous system has also been the technique used to halt incapacitating epileptic seizures and the symptoms of other movement disorders like Parkinson’s disease. In this procedure, electrodes are implanted directly in the skull, a method known as deep brain stimulation, or DBS. The same technique has been adapted to achieve direct electrical stimulation of the spinal cord, a procedure which recently allowed people whose spinal cords had been completely severed to walk again. In the future, however, medical neurotechnology will not only send electrical impulses to the brain and spine, but it will also receive signals in turn, recreating the bidirectional communication that truly takes place between the nervous system and every organ in the body. Thus, current research is progressing in integrating the brain control of the prostheses or of the body itself with the transmission of sensory signals back to the brain.
However, in this fusion of technology and brain, we need to proceed with caution. Given that consciousness—shaped by thoughts and by the sensations from the outside world that our nervous system receives—resides in the brain, how do medical neurotechnologies affect patients’ perception of themselves? Bioethicists have been pondering this question since the turn of the century, as some patients who receive deep electrical stimulation experience personality changes. Neurotechnology extends the functional limits of the brain, but it is important not to overstep ethical boundaries.
Comments on this publication