More than half of our body is not human. Only 43% of the cells that make up the human body are from our species. The rest are microscopic organisms that live in places like our skin or in large colonies that make up the intestinal flora. This is the microbiota, an enormous, very personal population, with such a unique composition that we believed it was written in the genes of each individual. However, a surprising study has just dismantled that belief and could bring about a medical revolution, because that microbial universe that we carry within us affects almost every aspect of our health, from allergies to mental illness, from body weight to cancer.
Researchers from the Weizmann Institute of Sciences in Israel have concluded that the genetics of the human host plays a very minor, almost residual, role in the composition of each microbiota. This means—in the words of Professor Eran Segal, one of the authors of this macro-study—that: “our microbiota could be a powerful way to improve health. We cannot change our genes but now we know that we can act on, and even remodel, the composition of different colonies of bacteria that are housed in our body.”

Mostly made up of bacteria—but also archaea, fungi or viruses—the microbiota can vary greatly from one individual to another, although it’s more similar among individuals of the same family, and also from the same region or population. Thus, everything pointed to the notion that the microbiota was determined fundamentally by genes, which define the particular conditions of each environment colonized by microorganisms, and it would be those internal conditions, such as the acidity of the gastric juices or the pH of the skin, that make certain microbes adapt better and proliferate with greater success. But now we know that 98% of the composition of the microbiota of each person is determined by external factors, mainly diet and lifestyle habits.
This revolutionary finding coincides with the boost that the US government has given to research in this field, with a strategic plan of five years (2018-2023) that will finance studies not only on the implications of the microbiota for health, but also for biosecurity, the environment or the development of new forensic techniques to solve crimes. This new plan is added to the one that in 2007 started a scientific race to get to know our microbial companions better by cataloguing all the genes of this set of organisms—which is known as the microbiome.
Intestinal flora that control the anxiety
Since then, the Human Microbiome Project has led to significant discoveries, such as the finding that the bacteria of the flora of the intestine exert a remote action on the brain—it is believed this occurs through the vagus nerve that connects both organs—regulating the production of proteins that control the anxiety states. “The way we think or how we feel could be controlled by our intestinal microbial flora,” explains Gerard Clarke, one of the co-authors of that study.
Accordingly, the presence or absence of certain intestinal bacterial populations determines that we suffer from (or are more prone to) diseases and mental disorders, from depression to autism. The effect is so much so that Paul Cyran, professor of neuropharmacology, “has coined the term psychobiotics to refer to the involvement of the microbiota in mental health.”
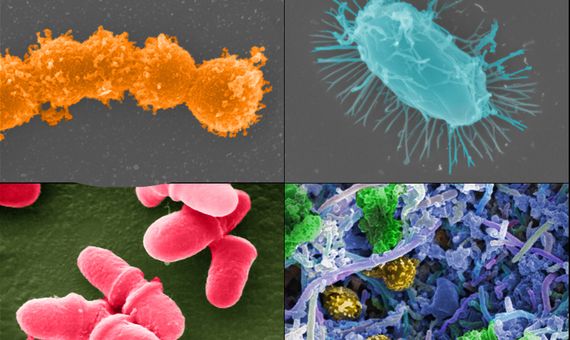
The Human Microbiome Project provided the first glimpse of the microbial diversity of healthy humans and is exploring the possible relationships between particular human diseases and the microbiome. Clockwise from top left: Streptococcus; microbial biofilm of mixed species, from human body; Bacillus; Malassezia lopophilis
It has also been discovered that certain bacteria that colonize our skin produce a compound that slows the synthesis of DNA in tumour cells, and thus the individuals that carry them are more protected against the development of skin tumours, a finding that further enhances the “potential of the microbiome to influence diseases,” in the words of Lindsay Kalan, biochemist at the University of Wisconsin-Madison. But perhaps this transcendental role of the microbiota (and its microbiome) in our health should not be so surprising. As Cyran points out: “All human evolution and all the systems of our body have coevolved with their microbial hosts.”
A complex ecosystem with a delicate balance
At the individual level, it is right at birth, when we are exposed to the outside world, when each person begins to acquire his or her own microbiota, which is shaped to a large extent during the first three years of life. From then on it tends to stabilize, but it will continue to change throughout life depending on external factors such as diseases, medications, environmental conditions, weather, stressful situations, hygiene habits or changes in diet. Along these lines, another recently published study has shown that adopting a diet rich in fibre induces the proliferation of certain bacterial strains that interact with the organism, favouring the production of insulin and thus reducing blood sugar levels, thereby decreasing the risk of suffering from diabetes.
But the fact that the microbiota can be changed does not mean that it is simple. The microbial flora and fauna of each individual constitutes a complex ecosystem with a delicate balance. Any subtle change can shake it up and in turn bring about a series of unwanted or unexpected changes, hence the importance of studying the microbiome holistically. As explained by Tim Spector, professor of genetic epidemiology: “It’s like a forest. You could have a fern that makes you very happy, but if you don’t have much diversity in your forest, it will be harmful to the substrate.” This is why it is “important to understand how the microbiome interacts with its human host before beginning to manipulate it to treat ailments and diseases,” says Lindsay Kalan.
In any case, the researchers involved in their study are hopeful that in the future they can act on the composition of the microbiome and consequently on health through pre- and probiotic foods, as well as by modifying life habits and environmental conditions.
Comments on this publication