Ununtrium, ununpentium, ununseptium and ununoctium. These are convoluted terms, but they are only provisional systematic names; soon they will be replaced by definitive ones, as science gave the official welcome to these four new chemical elements on December 30, 2015. Their temporary names refer to their atomic numbers (Z), or the number of protons: 113, 115, 117 and 118, respectively. With these four new additions, all the spaces in the seventh row of the periodic table of elements have been filled.
Chemistry students will now have much more material to memorize than in the time of Dimitri Mendeleev (8th February 1834 – 2nd February 1907), the Russian scientist who, in 1869, played cards with the 63 elements known to date and established the first version of the periodic table, which since then has continued to expand with a steady trickle of new members. Through to the arrival of flerovium (114) and livermorium (116), named in May 2012, the work of chemists has been becoming increasingly difficult; francium (87), discovered in January 1939 by Marguerite Perey, marked the end of the era of discovering new elements in nature. Two years earlier, the era of synthetic elements had been inaugurated with technetium (43), confirmed by scientists at the University of Palermo in December 1936.
Technetium, so called precisely because of its artificial origin, is one of several elements that were created by synthesis, before also being discovered in nature. But as atoms become heavier, unstable radioactive elements are obtained that can only be produced in the laboratory and decompose rapidly, sometimes in infinitesimal fractions of a second.
The strict boundary between the natural world and the laboratory is marked by plutonium (94), the heaviest element with isotopes stable enough to be found in the earth’s crust. Above this, americium (95) and curium (96), both created in 1944, opened the region of the periodic table that belongs exclusively to the realm of the laboratory or, at most, to experiments or nuclear accidents brought about by humans.
Heavier projectiles
Scientists are able to synthesize these superheavy elements by shooting atoms against one another in the hope that they fuse together, something that happens only once in billions of collisions. This is how the limit of element 118 has been reached, the heaviest of the recent arrivals. For example, element 117 was obtained by scientists from the United States and Russia bombarding a sample of 22 milligrams of berkelium (element 97) with ions from the isotope 48 of calcium for 150 days in the heavy ion accelerator of the Joint Institute for Nuclear Research in the Russian city of Dubna. In turn, berkelium took 250 days to be obtained from the Oak Ridge National Laboratory, USA. And all this effort managed to produce just six atoms of element 117, which disintegrated in just a few milliseconds.
How to discover a new element. Credit: LLNL
Therefore, it might be expected that exceeding the new boundary of the periodic table to the eighth row would be nothing more than unthinkable. Calcium bombardment has uncovered six new elements, but as physicist David Hinde, director of the Heavy Ion Accelerator at the Australian National University, shared with OpenMind, “the difficulties in going beyond element 118 is that the use of a Ca-48 projectile, which has many favourable properties, is no longer possible; heavier projectiles need to be used.”
However, Hinde is one of the men who may hold the key to advancing to the next box in the periodic table. He has recently studied the use of other projectiles in collaboration with the Superheavy Elements Group at the University of Mainz and GSI Center in Germany. The problem with using larger caliber projectiles, he explains, is that they “reduce the yield of superheavy elements by a factor or 10 or more.” Among the possibilities cited by Hinde are titanium-50, chromium-54, iron-58 and nickel-64.
For his part, physicist Jadambaa Khuyagbaatar, who belongs to the German group that collaborates with Hinde and who directed the confirmation experiments of element 117, is optimistic: “The results give us good hope to synthesize at least the next two elements, 119 and 120,” although “the experiments might take longer than for synthesis of elements 114 to 118,” he advises OpenMind. And according to Hinde, it may not be enough just to change the bullets, but perhaps more powerful guns will also be required: “New accelerators with higher flux of beam particles will help to overcome this disadvantage.”
Islands of stability
Another difficulty is the increasing instability of the heavier elements that hardly leave researchers with enough time to study their properties. But fortunately it seems that it is not always the case. In the 1960s, Nobel Prize-winning chemist Glenn T. Seaborg suggested that in the higher atomic numbers “islands of stability” might appear. The hypothesis is based on the idea that the protons and neutrons in the nucleus are arranged in layers of energy with a particular capacity; maximum occupancy of these layers provides “magic numbers” that are more stable than their lighter atomic neighbours. The prediction holds for the isotope 208 of lead, with double the magic number for protons (82) and neutrons (126), and which is indeed the heaviest stable nucleus. Predictions vary for the next possible magic number of protons, but some scientists place it at element 120, or unbinilium.
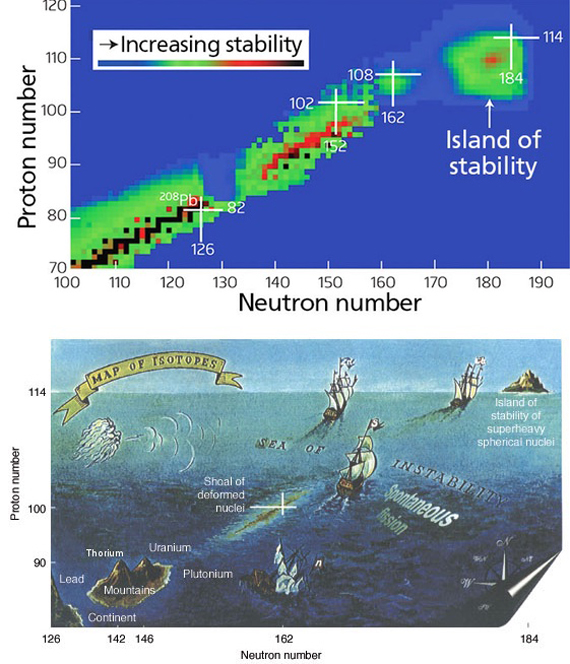
For Hinde, conquering this island might be a complicated undertaking, “or impossible, depending on where the center is.” However, the hidden treasure in that place could make up for the effort: “It is unlikely that these nuclei in the centre of the island are actually stable, but should have lifetimes measured in many years, rather than seconds or less as current superheavies have.” And according to Khuyagbaatar, at least we have made landfall. “We are still far from the center of the island; however, its shoreline has been reached.”
Comments on this publication