Introduction
Nearly everyone on the planet has by now encountered the words “global warming.” They appear almost daily in our newspapers and on television. Dozens of books on this subject and countless magazine articles appear each year. I hold the perhaps dubious distinction of having been the first to use them in print. In 1975, I published a paper in Science entitled “Climate change: Are we on the brink of a pronounced global warming?” In it I offered an explanation as to why, despite a continuing rise in the atmosphere’s CO2 content, Earth temperature remained very nearly constant from 1940 to 1975. My hypothesis was that an extension of the 80- and 180-year periodicities in air temperature recorded in the Camp Century Greenland ice core in the centuries before the Industrial Revolution suggested that the expected man-induced CO2 warming had, by chance, been compensated by a natural cooling. Further, if this were the case, the Earth was poised for a turn around, for the natural cooling was about to turn the corner and become a natural warming. If so, nature would join forces with made-made CO2 and the Earth would warm. As it turned out, my prediction was right on. A year after my paper was published, the Earth began warming and has continued to do so right up to today. But to my chagrin, the 80- and 180-year cycles so prominent in the record from northern Greenland have not shown up in any subsequent climate record (including those from ice cores in central and southern Greenland).
Figure 1:
A) Diagram reproduced from my 1975 Science paper. The solid curve is the global temperature record available to me at that time. The dotted curve is my guess regarding the temperature rise attributable to manmade CO2. The light dashed curve is my guess regarding the natural fluctuations in global temperature based on the 80- and 180-year cycles seen in the oxygen isotope record from the Camp Century Greenland ice core. The bold dashed curve is the sum of my two guesses.
B) The updated global temperature record. Although my prediction turned out to be qualitatively correct, the 80- and 180-year cycles on which it is based have not shown up in any other long climate record.
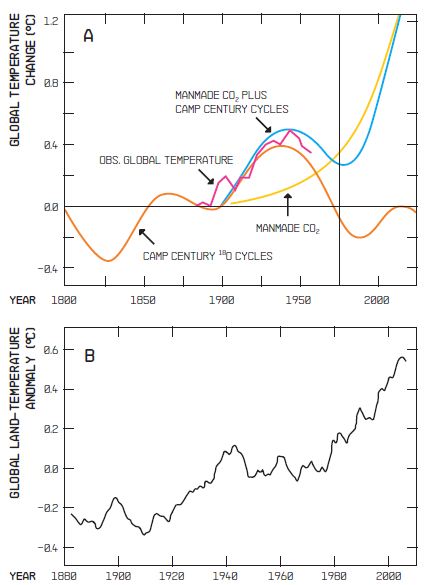
To be sure, the naysayers point to this unexplained temperature plateau as support for their claim that global warming is little more than a tempest in a teapot. With equal vigor, they point to the advances of mountain glaciers that took place during the seventeenth and nineteenth centuries and to the warm condition that allowed the Vikings to colonize Greenland a millennium ago as evidence that CO2 is not to blame. Rather, they would like to believe that it’s all natural, perhaps driven by the Sun.
Those of us who firmly believe that CO2 has warmed and will continue to warm the planet, look upon these past temperature changes as background fluctuations that will surely continue. Further, we believe that the impacts of CO2 and other so-called greenhouse gases are not yet large enough to have pushed us beyond the reach of these natural fluctuations. But we have likely experienced the last of natural plateaus and downturns. Until we rein in the rising greenhouse gases, these natural ups and downs will likely only serve to modulate the steepness of a continuing warming.
Greenhouse Physics
Physics demands that the increases in the concentrations of gases like CO2, which are capable of capturing quanta of outgoing Earth light, must warm the planet. The reason is that when these gases reemit the captured energy, only one half is sent toward outer space. The other half is sent back toward the Earth’s surface. As a result, in order to balance the energy received from the Sun, the Earth must compensate by emitting more earth light. To do so, the Earth’s surface must become warmer.
Were CO2 and the other manmade greenhouse gases (i.e., methane, nitrous oxide, CFCs…) the only players, our impact on Earth temperature would be not nearly so worrisome. But a powerful feedback occurs. As the planet gets warmer, the vapor pressure of water rises. Because they are so much more abundant than CO2 molecules or those of any other greenhouse gas, water molecules dominate the atmosphere’s ability to capture outgoing Earth light (i.e., infra-red rays). For each degree Celsius Earth temperature rises, the vapor pressure of water increases by 7% and consequently the amount of water vapor in the atmosphere increases by a similar amount. This mushrooms the primary impact of manmade greenhouse gases by a factor of about three. So, a doubling of atmospheric CO2 content from its 280 parts per million pre-industrial value to the 560 parts per million value we are likely to reach by this century’s end is projected to warm the planet by about 3.6°C instead of the 1.2°C rise expected from CO2 alone.
To my knowledge there is only one scientist with unquestioned credentials who denies this water vapor enhancement of the CO2 warming. He is Richard Lindzen, a renowned atmospheric physicist at MIT. While not questioning the basic physics described above, he concludes that, rather than enhancing, the warming water vapor will work to reduce it. His argument involves a redistribution of the atmosphere’s water vapor; he admits that more will be present in the tropical atmosphere but hypothesizes that less will be present in the extra tropical atmosphere (i.e., above its drylands). Upwelling of high moisture-content air in the tropics creates a dense cloud cover that greatly impedes the loss of Earth light. By contrast, down welling of low moisture content air in the extra tropics provides a major escape hatch for Earth light. So Lindzen would put the extra water vapor where it would be least effective and by drying the air in the extra tropics, he opens the escape hatch even wider. The problem is that no one, including Lindzen, has created a fully-fledged computer simulation that accomplishes this feat. All such simulations lead to a more nearly uniform increase in water vapor and hence a large enhancement of the primary warming.
While Lindzen’s reputation as a scientist remains intact, his battle against mainstream science wears thin. Further, the fact that he will argue with equal forcefulness that there is no proof that cigarette smoking is linked to lung cancer makes it tempting to write him off as a contrarian.
Aerosols and Clouds
Bedeviling attempts to understand what is going on today and what the future holds are the impacts of man-made aerosols on the Earth’s radiation budget. These aerosols both reflect away incoming sunlight and absorb outgoing Earth light. The sulfur released by burning coal ends up as light-colored sulfuric acid aerosols, which act primarily as solar reflectors and hence tend to cool the Earth. The carbon released during biomass burning ends up in dark-colored soot, which, like greenhouse gases, captures Earth light and hence tends to warm the Earth. Unlike greenhouse gases, which have well-defined optical properties and are uniformly distributed throughout the atmosphere, aerosols have complex optical properties and are concentrated in the regions adjacent to their sources. Because of this, a large uncertainty exists regarding their contribution to man-induced climate change. While opinion has it that cooling by sulfate currently outweighs warming by soot, there is concern that the ongoing increase in soot emissions will turn the tables and aerosols will soon enhance rather than impede warming.
In thinking about this one has to keep in mind that as aerosols remain aloft for only days to weeks before being purged from the atmosphere by rainfall. By contrast, CO2 molecules will remain airborne for hundreds of years before being taken up by the ocean. Hence as time goes on, the importance of CO2 relative to aerosols will become ever larger.
In addition to their direct role as perturbers of the Earth’s radiation budget, aerosols have an important indirect role. They serve as condensation nuclei required to form raindrops. The more condensation nuclei present in a cloud, the more droplets that will form. As a fixed amount of water is available for condensation, the droplets will be smaller. Importantly, this makes the cloud more reflective. As shown, a dramatic demonstration of this indirect impact can be found in the bright trails created by the smoke rising from ships passing beneath the low cloud cover.
Taken together, our inability to assess the contribution of natural climate fluctuations and to assess the contribution of aerosols makes it impossible to evaluate whether the warming CO2 and other greenhouse gases have created is consistent with the expectation.
Future Prospects
Each year we currently burn fossil fuels containing about 7 gigatons of carbon. All the CO2 thus produced is released to the atmosphere. A bit more than one half remains airborne. The remainder is taken up by the ocean and by the terrestrial biosphere. Although the ocean uptake is well understood, that by the terrestrial biosphere remains a mystery. It is so large that it more than compensates for CO2 release associated with deforestation (about 1 G ton C/yr). The result is that the CO2 content of the atmosphere is currently rising at the rate of 2 ppm/yr. As of the end of 2009, it was about 390 ppm which is 110 ppm higher than its pre-industrial value.
Figure 3: Charles David Keeling’s record of the CO2 content of the air at high elevation on the island of Hawaii. The wiggles reflect the season cycle of photosynthetic uptake and respiratory release of CO2 by Northern Hemisphere plants. The steepening of the record reflects the steady increase in fossilfuel burning.

Under the Kyoto Accord, the industrial nations (but not the USA) have agreed to make modest reductions in their carbon burning. But these small reductions are being more than eclipsed by greatly expanded energy use in China, India, and other traditionally poor countries. As this situation will very likely prevail during the next several decades, the expectation is that the rate of CO2 rise will increase to at least 3 ppm/yr. At 3 ppm/yr, CO2 would increase by 150 ppm in the next 50 years bringing the total to 540 ppm or only 20 ppm short of double the pre-industrial level (560 ppm).
What should we do?
It is clear to everyone that increased efficiency in energy use is not only essential but also a win-win effort. And, of course, we must go all out to develop and implement affordable non-fossil fuel energy sources. But, regardless of how effective these measures are, the world is in for a change in climate that will alter the pattern of rainfall and melt ice caps. Hence, we must prepare to deal with these changes.
I fear that conservation and alternate energy alone will not be capable of bringing the rise in CO2 to a halt for this requires that we reduce our current CO2 emissions by tenfold. As around 85% of the world’s energy is currently derived from fossil fuels, this means that stopping the CO2 rise will involve replacing virtually all our energy generation systems (including those used by automobiles, ships, and airplanes). Only through very large-scale use of nuclear and photovoltaic power would this be possible. Wind, geothermal, solar thermal, and vegetation likely lack the potential to become the dominant players.
This being the case, it is essential that we prepare to capture and store CO2. Even if a minor miracle occurs and we do find a way to obtain the world’s energy without burning carbon, there will very likely be a call to bring the atmosphere’s CO2 content back down. I say prepare because there is much we need to learn about both the capture and the storage of CO2.
CO2 Capture
Most of what’s been written about CO2 capture involves the exhausts from electrical power plants. The consensus appears to be that rather than stripping it from the hot stack gases of conventional coal-fired plants, it would be better to build what is called coal gasification plants. In these plants the coal is treated with steam converting it to carbon monoxide and hydrogen. The hydrogen is then used to generate electricity in a fuel cell and the CO is converted to CO2, which is captured, liquefied, and piped to a storage site. However, to date no such plant is operative.
My colleague Klaus Lackner has convinced me that direct capture of CO2 from the atmosphere is a superior strategy. He points out that despite the low concentration of CO2 in air the cost of direct capture is comparable with that from electrical power plants. The reason is the energy cost is documented by a single step in the process, namely the removal of the CO2 from the capture medium.
Over the last 6 years, Lackner and his associates have developed an economically feasible means of air capture. The cost would be about 30 dollars a ton of CO2 (equivalent to an increased cost of 25 cents per gallon for gasoline or 2 cents per kilowatt hour for electricity). Further, as the collectors would be placed close to the storage sites, the cost of piping CO2 from power plant to storage site would be largely eliminated.
Lackner’s devices would be modular. Each would retrieve one ton of CO2 per day from the atmosphere (i.e., the daily amount created by 20 automobiles). The components of each unit would fit into a standard shipping container. The cost of each unit would be about that of an automobile. Hence a 5% surcharge on automobile purchases would pay for the manufacture of these devices.
The capture medium used in Lackner’s device is a plastic fiber with built-in ligands (positively charged molecules). When exposed to air, H2O molecules occupying the ligand sites are replaced by CO2 molecules. When the CO2-loaded fibers are then subjected to steam at 40°C, H2O molecules replace the CO2 molecules. This cycle has been repeated hundreds of times without any diminishment of the fiber’s uptake capacity. Nor do the fibers deteriorate from exposure to urban air.
Lackner envisions that the prototype device once constructed would be as shown in Figure 4. Thirty mattress-size collectors would be assembled in a circle above the unit housing the vacuum chambers. Thirty more would reside in the chambers. Using an elevator, the fiber packs would be rotated from exposure to air to treatment with water vapor. Once loaded with collectors, the chamber would be evacuated. Steam would then be introduced. Following this, the CO2-residual steam mixture would be pumped out and compressed, liquefying much of the residual water vapor. The remaining vapor would be removed on a drying agent and, finally, the dry CO2 would be further compressed until it liquefied. It would then be piped off to storage.
Lackner’s modular strategy would allow for continuing improvement of the unit’s design. He would start with the equivalent of a 1934 Ford and eventually end up with that of a 2009 Toyota!
CO2 Storage
Whether captured in electrical power plants or retrieved from the atmosphere, the CO2 must be stored. The ideal would be to react the CO2 in a chemical plant with magnesium (Mg) extracted from olivine and pyroxene minerals in ultrabasic rock (or its serpentinized equivalent). The Mg CO3 manufactured in this way would last forever. But until some means to reduce the very large energy cost associated with this approach has been found, lower cost storage options will have to be used. Four archives have been suggested: 1) as liquid CO2 in aquifers whose pore space is currently filled with hyper saline water, 2) as a solid CO2-H2O clathrate in lakes beneath the Antarctic continent, 3) as HCO3 in the deep sea, 4) as Mg and Ca bicarbonate ions or Mg and Ca carbonate minerals in basalt or ultrabasic rock. To date, none of these repositories has been adequately tested: questions about the costs and environmental consequences remain for each.
Figure 5: Two possible modes of CO2 capture and four of CO2 storage.
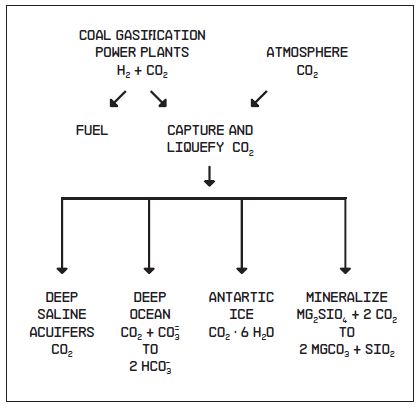
Aquifers
The most talked about option is storage in hyper saline aquifers. These aquifers are widespread at one to two kilometers depth in continental interiors (and beneath shallow marginal seas). As the host rock is sandstone, there would be little opportunity for neutralization of the CO2. It would remain in liquid form. The advantage of this option is that these aquifers are not under international control as are the deep sea and Antarctic ice cap. The disadvantage is that the people living above them are bound to have safety concerns, and, where the law permits, likely claim ownership.
Basalt
Several large regions of the planet are covered with thick sequences of basaltic lava flows. These flows are thought to have formed when giant plumes of hot rock originating at the core-mantle boundary ascended to the Earth’s surface. Prime examples are found in Brazil, in India, in Siberia and in the northwestern USA. It has been proposed that if CO2 dissolved at high pressure in water were injected at depth in these flows, it would dissolve pyroxene and the olivine minerals in the basalt releasing magnesium ions. These positively charged ions would immediately react with CO2 molecules converting them to bicarbonate (HCO3) ions, and in this way, permanently immobilizing them. As the reaction proceeded, carbonate (CO3) ions would form and eventually a MgCO3 solid would precipitate.
Of the many questions regarding this approach, the major one is the extent to which the CO2 would leak back to the atmosphere (through the ubiquitous fractures) before it was able to react with the host rock. In order to evaluate the competition between reaction and leakage, an experiment is currently being conducted in Iceland whose terrain consists entirely of basalt.
Deep ocean
Although in my estimation storage of CO2 in the deep Pacific Ocean is certainly an appealing option, many voices oppose it. Of these, Greenpeace is the most vocal. My proposal is to pre-load this vast reservoir with roughly the amount of CO2 which will get there on its own over the next few hundred years. In other words we would short circuit the delivery of CO2 to this vast reservoir. Most of this CO2 would be immobilized by reacting with resident carbonate and borate ions to form bicarbonate ions. Roughly 200 gigatons of C as CO2 could be stored without raising the partial pressure of CO2 in the water more than it has been raised to date in the atmosphere. We know from 14C measurements on the bicarbonate in deep Pacific waters that its isolation time there is about one millennium. We also know from the distribution of 3He released from ridge crests that on this time scale the entire deep Pacific becomes well mixed. The best means of addition would be to pipe the liquid CO2 down to more than 3.5 km, for at this depth it becomes denser than sea water. We also know that the liquid CO2 would react with water to form a clathrate slush (7H2O4 + CO2) which would sink to the sea floor. Of course, the clathrate would, on the time scale of decades, be dissolved into the surrounding sea water.
In my mind, the damage to the benthic creatures would be localized to the chemical halos in the vicinity of the injection sites. In order to assess extent of this damage, pilot experiments must be conducted. Several tons of liquid CO2 would be delivered to the abyss from a drilling vessel. Sensors would be deployed and tracers added to the CO2 so that its dispersal could be tracked. Also cameras would be placed to observe the reaction of the abyssal swimmers.
Antarctic lakes
Somewhat surprisingly from a storage point of view is that the best storage sites lie beneath the Antarctic ice cap. Were the liquid CO2 piped into any one of the hundred or so lakes underlying the ice cap, it would react with water and form a CO2-H2O clathrate, which would sink to the lake’s rock floor. Lacking carbonate or borate ion, the waters of these lakes would not have the capacity to redissolve the clathrate. Hence it would remain in solid form until thousands of years in the future the slow motion of the ice carried it to the edge of the ice sheet where it would be discharged into the sea.
The heat given off during the formation of the clathrate would be dissipated by melting ice from the lake’s roof. It turns out that the amount of water generated in this way would roughly balance the consumption of water by clathrate formation. Hence, the volume of water in the lake would not be depleted.
But, of course, those concerned with preserving the pristine state of the Antarctic plateau would surely howl. Further, the existing international law against mining in Antarctica would likely prevent the construction of the apparatus needed to capture CO2 and pump it beneath the ice cap, and, of course, also the construction of the housing, etc., for the people who installed and operated this equipment. But, as we face an extraordinary challenge, no option should be ruled out without being given careful consideration.
Ultrabasic lock
The Earth’s mantle is made largely of three elements: magnesium, silicon, and oxygen. Surprisingly, slivers of mantle material pierce the crust and outcrop at many places. For example, much of the bedrock in Oman is ultrabasic rock or its serpentinized equivalent. The magnesium in these rocks is a tempting ingredient for permanent sequestration of CO2 as the mineral MgCO3. Two approaches have been considered. One is to mine the rock, dissolve it in a factory and then mate the magnesium with CO2. The products (magnesite and opalline silica) would then be dumped back in the hole created by the mining operation. Unfortunately to date, no one has figured out how to do this at an acceptable energy cost. Another idea is to do it in situ. CO2 would be injected into the rock. As its reaction with the rock gives off both heat and also increases the volume of the rock (and as a result opening cracks) perhaps a self-sustained reaction could be created. Again much research would be required to determine whether this process could be harnessed.
Countermeasures
What could be done if the drive to squelch the buildup of CO2 in the atmosphere fails? In the late 1960s, a Russian meteorologist, Mikhail Budyko, proposed that the input of solar radiation could be reduced by loading the stratosphere with SO2. There, the sulfur dioxide would react with an oxidant to produce sulfuric acid aerosols that would reflect away sunlight and in this way cool the Earth.
In the mid 1980s, John Nuckolls, a physicist at Livermore National Laboratory, and I decided to take advantage of new information and update Budyko’s scenario. Model simulations had shown that, in order to compensate for a doubling of atmospheric CO2 content, 2% of the Sun’s incoming radiation would have to be reflected back to space. As H2SO4 aerosols back-scatter only 10% of the solar rays that strikes them, in order to reduce insolation by 2%, these aerosols would have to intercept 20% of the incoming sunlight. This would require the sulfuric acid aerosols produced from about 30 million tons of SO2. As the aerosols would remain aloft in the stratosphere for about one year, 30 million tons would have to be sent up annually.
A call to Freeport Sulfur Company provided the yearly SO2 cost (i.e., about 10 billion 1980’s US dollars). A call to Boeing indicated that the purchase and operation of the fleet of seven hundred 747 aircraft needed to carry the SO2 to the atmosphere would involve an annual cost of 20 billion 1980’s US dollars.
Nuckolls and I put together a paper entitled “An Insurance Policy Against a Bad CO2 Trip.” In addition to the above, it included some words regarding the environmental side effects of such a remedial action (i.e., additional acid rain, ozone reduction, etc.). We then sent this draft around to several prominent scientists (Frank Press at the National Academy of Sciences, Bert Bolin who at a later date headed the IPCC, Jerry Makman of NOAA’s Geophysical Fluid Dynamics Laboratory, and others). Each advised us not to submit the paper for publication. One reason was their fear that it would provide an excuse for government inaction. We heeded their advice. The only published record of our thoughts was a short piece in The Daily Telegraph [Figure 6]. It wasn’t until over a decade later that Nobel Laureate Paul Crutzen published an article on this subject.
My fear is that we will not move at a fast enough pace on CO2 emissions reduction and the planet will become sufficiently warm that a majority of nations will opt for the SO2 bailout. As the aerosol Band Aid will cost ten times less than any scenario for stemming the CO2 buildup, the temptation to adopt this path will be large.
The Next Twenty years
It is my view that it will take at least two decades before a meaningful international agreement can be reached. Although the realization that we must do something has become widespread, there remains a deep resistance to anything that might be viewed as a carbon tax, and a deep suspicion that the terms of any binding treaty would be violated by our economic competitors. But, as the planet warms, the ecologic changes, the parching of drylands, and the melting of ice will become ever more evident. These changes will increase the pressure to bring the CO2 rise to a halt and hopefully force the world’s political leaders to sign a treaty that has teeth in it.
If this scenario is correct, then it is of the upmost importance that the intervening time be used to further the development both of methods for the production of non-carbon energy and of methods for the capture and storage of CO2. Although industry has a strong profit incentive to do the former, this is not the case for the latter. Hence, government will have to step in. As time is of the essence, we must not let the next two or three decades slip doing as little as we’ve done during the last two or three.
As a citizen, I wish that I could live to observe how the world responds to this huge environmental challenge. As a scientist, I wish I could live to observe the impacts of what the late Roger Revelle termed man’s greatest geophysical experiment. But, alas, as I’m approaching my 78th birthday, I surely won’t be around when the big crunch arrives!
Comments on this publication