This is an improbable story. Improbable because until less than a decade ago it seemed impossible to be able to see how electrons move in a molecule, breaking and forming their bonds, in other words, pulling the threads of chemistry. In the subatomic world everything happens incredibly fast, at speeds measured in attoseconds—a billionth of a billionth of a second (that is, 10-18 seconds). And on that scale, a second is a virtually infinite amount of time.
Improbable also because to see and record the movement of something so small and so fast you need huge installations and supercomputers calculating for years. Improbable, in short, because a discovery rarely comes along that can change the way chemistry is practiced. This is, therefore, a story that requires a bold imagination.
In 2001, a technological breakthrough occurred that altered this improbability. Researchers at the Max Planck Institute of Quantum Optics, in the German city of Göttingen, generated with super-fast lasers the first pulses of light that lasted just attoseconds. For humans this is an irrelevant interval of time, but in those very brief instants is when electrons display their natural rhythm. For the first time, the necessary light source was available to “see” them and, perhaps, to record them.
The first attosecond camera
Eight years later, a team led by Fernando Martín, professor of chemistry and physics at the Autonomous University of Madrid, Marcus Vrakking, director of the Max Born Institute in Berlin and Mauro Nisoli, professor of physics at the Polytechnic of Milan, designed the first attosecond camera capable of seeing the movement of electrons in molecules. The first film gave us in real-time an intimate view of hydrogen, the simplest molecule in the universe.
The experiment was inspired by the camera that the Egyptian Nobel Prize winner Ahmed Zewail had designed to see the movement of the atomic nuclei, but with greater resolution. The camera works by sending a pulse of light lasting some attoseconds that irradiates a molecule and induces the movement of electrons. At intervals, also measured in attoseconds, another pulse of the same ultra-fast nature takes photographs of the movement, which are then projected in a concatenated way, creating the illusion of movement—like the train arriving at a station, which so amazed audiences of the first films of the Lumière brothers in 1896.
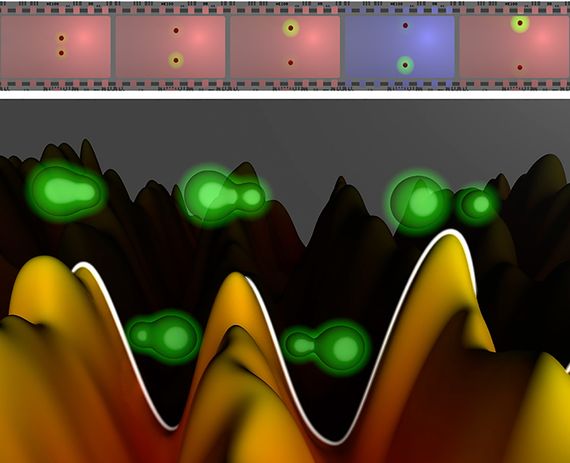
“The difference with a normal movie is that to film something that moves in times as short as attoseconds, you have to take pictures with exposure times that are of the same order. Otherwise they would come out blurry,” explains Martín.
Overcoming the technical complexity—these lasers occupy the entire floor of a building and have thousands of pieces and optical devices—was made possible by combining the talents of the three scientists: Nisoli was a pioneer in the development of one of the first attosecond light pulses, Vrakking an expert in molecular spectroscopy, and Martin leads one of the only two groups in the world capable of developing the visualisation tools needed, because the images that emerge from these cameras are not understood at all—they are only blurry spots.
“It’s a bit more complicated but the basic idea is the same as in 3D movies: if you don’t wear the special glasses you get at the cinema, the image looks blurred. We have to develop the equivalent of glasses to transform the images into something we understand,” Martín continues. These tools are obtained by solving the Schrödinger equation, which governs the atomic and subatomic world in the same way that Newton’s rules govern the macroscopic one. However, they are much more difficult to solve, especially in the case of molecules, and supercomputers are needed. Martín’s team used the MareNostrum supercomputer at the National Supercomputing Centre in Barcelona. The calculations took a year.
Control chemical reactions
Four years later, in 2014, Martín and Nisoli obtained the first film of a molecule with biological interest: phenylalanine, an essential amino acid. During the experiment, another unlikely effect appeared—in addition to seeing the movement of electrons in a more complex molecule, the scientists found that with these pulses of light they could, one might say, modify it at will. And now is when this story takes a turn that we can only imagine. Because the bonds between different atoms are broken or formed according to what the electrons say, if they moved in a different way they could break or form other bonds; in other words, the resulting chemistry could be completely different from what we know.
“The objective is to try to control chemical reactions at will, for example, forcing something to react because of a pulse of light that is going to change the movement of its electrons, or the opposite, that molecules that normally react spontaneously stop doing so,” explains Fernando Martín.
These discoveries are giving rise to a new way of doing chemistry based on the use of attosecond lasers and supercomputing, a term for which has already been coined: attochemistry. There is still much to be done until techniques emerge that can reach the laboratories. Meanwhile, several groups are now investigating its application in condensed matter such as graphene, the new material that some say is going to change the world.
Eugenia Angulo
Comments on this publication